In Antibody-Drug Conjugates (ADCs), antibodies serve as targeting agents that deliver drugs to cancer cells. This article focuses on the “Variable Region,” the key structure that enables antibodies to specifically recognize cancer antigens, and explains its critical role in ADC design.
Basic Antibody Structure: Constant and Variable Regions
Antibodies have a Y-shaped structure consisting of two main parts:
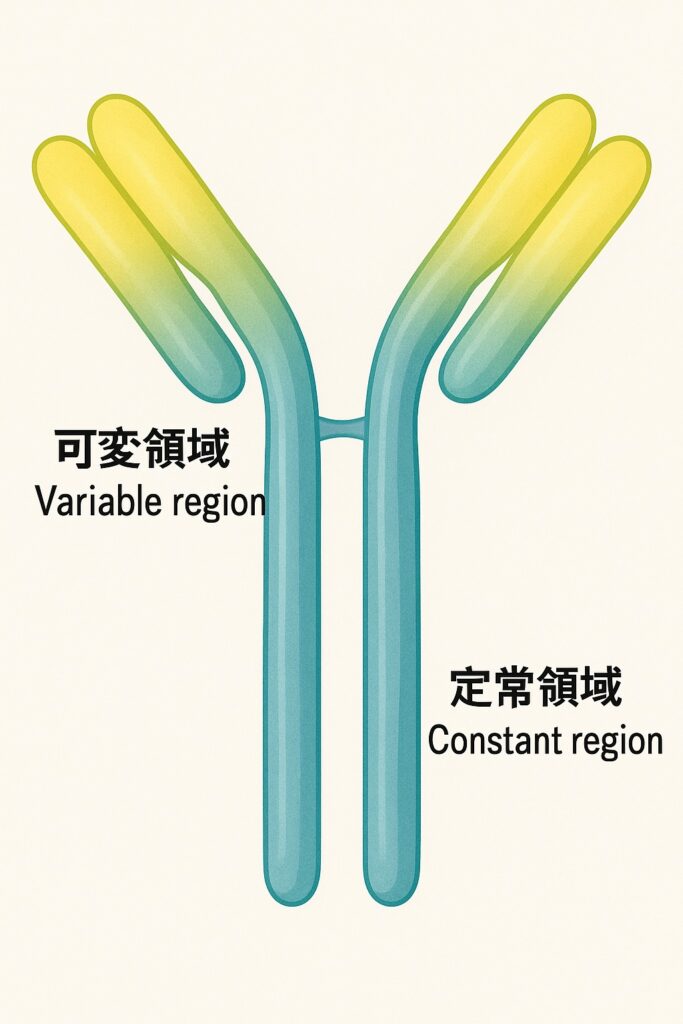
- Constant Region: Responsible for interaction with the immune system.
- Variable Region: Recognizes and binds to specific antigens—acting as the “eyes” of the antibody.
How Does the Variable Region Work?
For an antibody to “see” cancer cells, its variable region must match the three-dimensional structure of the target antigen precisely.
This region contains three short segments known as Complementarity Determining Regions (CDRs), which are responsible for the antibody’s high affinity to its target.
Why the Variable Region Is Crucial for ADCs
ADC design must balance specificity and efficacy. The variable region plays a central role in achieving this by:
- Cancer cell selectivity: Ideal variable regions bind only to antigens expressed on cancer cells, not on normal tissues.
- Internalization efficiency: Whether the antibody is internalized into the cell after binding often depends on the antigen recognized by the variable region.
Example: HER2-Targeted Antibodies
Trastuzumab, used in HER2-positive breast cancer, is a well-known antibody with a variable region specific to the HER2 protein. It also serves as the backbone for some ADCs.
Coming Next (Part 4): Linker Types and Cleavage Mechanisms
In Part 4, we will explore the “linker” that connects the antibody to the payload. You’ll learn which types are stable in the bloodstream and how they are cleaved within target cells.
▶️ See the full series here:














Comments