[Review] A Landmark Overview on Stem Cell Aging, Cell Stem Cell 2025
Introduction
As we age, one of the most fundamental biological changes is the decline in stem cell function. Stem cells are essential for maintaining tissue homeostasis and enabling regeneration after injury. When they age, not only does our regenerative capacity decline, but risks of chronic diseases and degeneration increase. In this fourth entry of the Aging, Rejuvenation & Regeneration series, we explore the five key hallmarks of stem cell aging as summarized in the 2024 Cell Stem Cell review “Hallmarks of Stem Cell Aging,” and consider how these insights may shape the future of regenerative medicine.
The 9 Hallmarks of Stem Cell Aging
- DNA Damage & Genomic Instability: Accumulated mutations impair self-renewal and differentiation.
- Epigenetic Alterations: Aging drives changes in DNA methylation and histone modifications.
- Metabolic Rewiring: Altered mitochondrial activity and energy balance shape cell fate.
- Loss of Proteostasis: Decline in protein quality control mechanisms causes aggregation.
- Chromatin Remodeling: Relaxed chromatin state disrupts gene regulation.
- Changes in Niche Communication: Aged microenvironments reduce stem cell activity.
- Inflammation: Chronic inflammation impairs stem cell functions.
- Senescent Cell Accumulation: Alters niche signaling negatively.
- Decline in Stem Cell Number and Potency: Loss of self-renewal and multipotency.
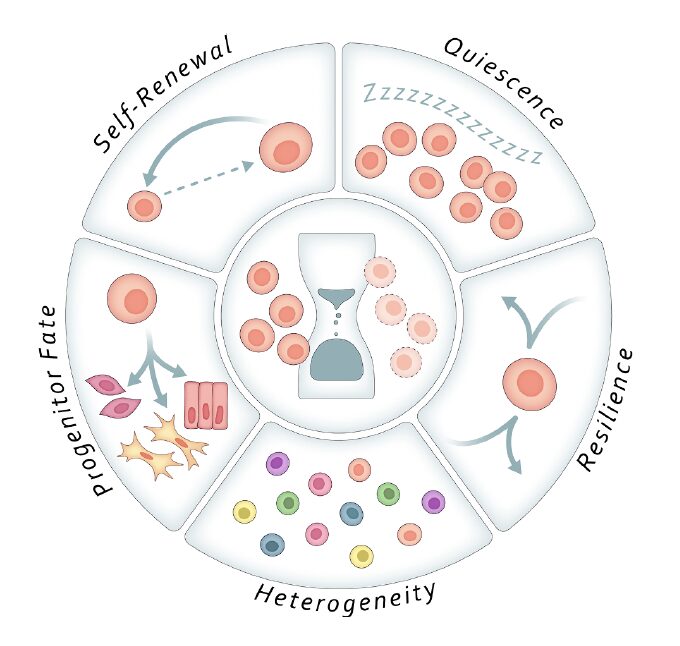
1. Deepening of Quiescence
With age, many tissue-resident stem cells tend to enter a state of “deep quiescence.” This condition is characterized by decreased sensitivity to external activation signals, making it harder for stem cells to exit dormancy and respond to regenerative cues. In practical terms, this results in delayed tissue repair, insufficient regeneration, and impaired response to damage in aging tissues.
2. Loss of Self-Renewal and Differentiation Bias
Aged stem cells often lose their capacity to self-renew and simultaneously develop biases in their differentiation fates. For instance, hematopoietic stem cells (HSCs) may increasingly produce myeloid cells over lymphoid cells. These shifts are believed to be driven by changes in transcription factor balance, epigenetic remodeling, and alterations in the stem cell niche microenvironment.
3. Decline in Stress Resilience
Young stem cells exhibit robust mechanisms for handling stress, such as efficient DNA repair and mitochondrial quality control. Aging disrupts these defenses: autophagy declines, reactive oxygen species (ROS) accumulate, and mitochondrial dysfunction becomes prevalent. These defects reduce the longevity and stability of the stem cell pool and increase the risk of senescence and apoptosis.
4. Altered Cellular Heterogeneity
Functional diversity within stem cell populations is vital for adaptability and resilience. However, aging skews this balance. Clonal expansion of specific subsets reduces overall heterogeneity, leading to a loss of adaptive potential and, in some cases, a predisposition toward malignant transformation or clonal hematopoiesis. In essence, aging narrows the regenerative “toolbox.”
5. Disruption of Molecular Signaling and Loss of Plasticity
Aged stem cells show altered responses to key signaling pathways such as Notch, Wnt, and TGF-β. These changes diminish their plasticity—the ability to flexibly respond to environmental cues and tissue demands. The result is impaired adaptation, reduced regenerative range, and often the promotion of chronic inflammation or fibrosis.
Therapeutic Interventions to Rejuvenate Aging Stem Cells
Understanding these hallmarks provides a blueprint for designing interventions aimed at rejuvenating stem cells. Strategies under investigation include:
- Reversing deep quiescence: Enhancing responsiveness to activation signals and mobilization
- Epigenetic reprogramming: Restoring transcriptional flexibility through chromatin remodeling
- Metabolic rebalancing: Improving mitochondrial function and promoting autophagy
- Anti-inflammatory modulation: Targeting age-related chronic inflammation (inflammaging)
These approaches aim not just to preserve cell number but to recover function, flexibility, and regenerative potential at the cellular and tissue level.
Author’s Perspective
The study of stem cell aging is evolving from passive observation to active intervention. Rather than merely cataloging functional declines, researchers are now seeking ways to preserve or restore stem cell “quality” through targeted therapies. This paradigm shift recognizes that aging is not a one-way process, and that interventions targeting quiescence, metabolism, and epigenetic state may hold the key to sustained regenerative capacity. As we refine our understanding of the stem cell aging network, we move closer to a future in which long-term tissue health and regenerative medicine converge.
Reference: Hallmarks of stem cell aging, 2025 July 03, Cell Stem Cell, Cell Press





Comments