CAR-T cell therapy has emerged as a groundbreaking treatment that specifically targets cancer cells. Among its forms, the “in vivo type” CAR-T—where the therapeutic process happens inside the body—is gaining attention as a next-generation approach. In this series, before diving into the cutting-edge developments, we start by explaining the fundamentals of CAR-T, using a real-life success story: the emotional journey of a young girl named Emily.
Table of Contents
- The Girl Who Moved the World: Emily’s Miracle
- Understanding the Mechanism of CAR-T Therapy
- What’s the Difference Between Ex Vivo and In Vivo CAR-T?
- Summary and What’s Next
1. The Girl Who Moved the World: Emily’s Miracle
In the early 2010s, a young girl was admitted to the Children’s Hospital of Philadelphia (CHOP) in the United States. Her name was Emily Whitehead. She was suffering from acute lymphoblastic leukemia (ALL), a type of blood cancer. Despite undergoing various standard treatments, her cancer kept returning.
At just six years old, Emily and her parents were told by doctors that all treatment options had been exhausted. In a final act of hope, they turned to an experimental immunotherapy called CAR-T cell therapy, which had just been developed by Dr. Carl June’s research team at CHOP.
CAR-T therapy involves collecting a patient’s T cells, genetically engineering them to recognize and attack cancer cells, and re-infusing them into the body. Emily received a clinical trial therapy called “CTL019.” In April 2012, the modified CAR-T cells were infused into her bloodstream, leading to a response far beyond expectations.
The initial side effects were severe—she developed life-threatening cytokine release syndrome and had to be moved to intensive care. Fortunately, thanks to quick thinking by the medical team, she was treated with an anti-IL-6 antibody and survived. A few weeks later, tests showed that her leukemia cells had completely disappeared.
Emily’s dramatic recovery drew international media attention and rapidly boosted global awareness of CAR-T therapy. Today, Emily is a university student and continues to advocate for cancer awareness.
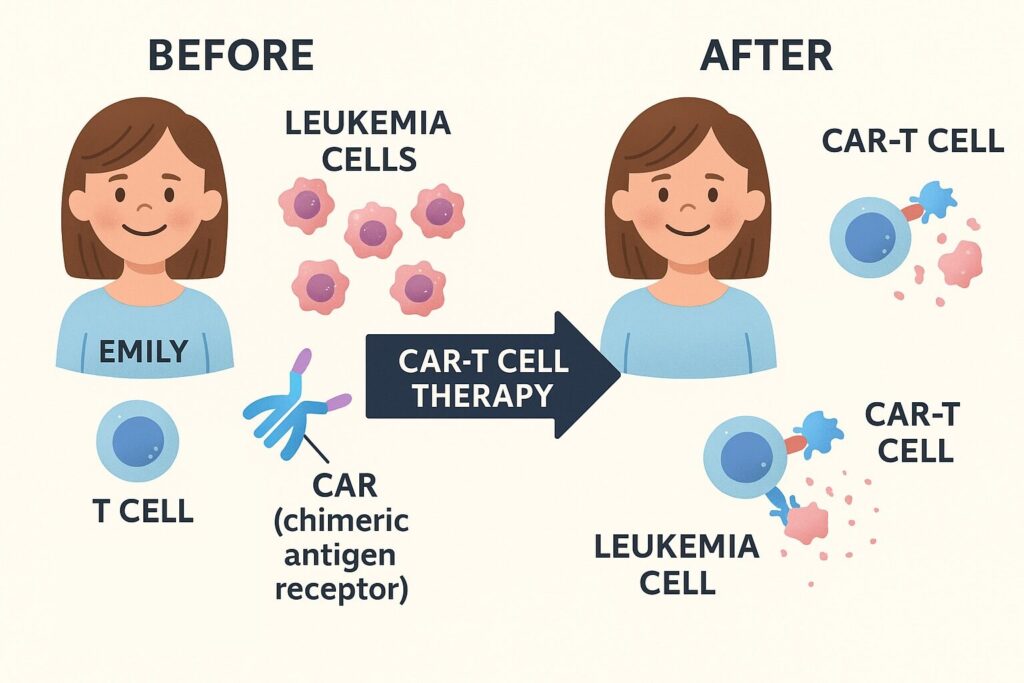
2. Understanding the Mechanism of CAR-T Therapy
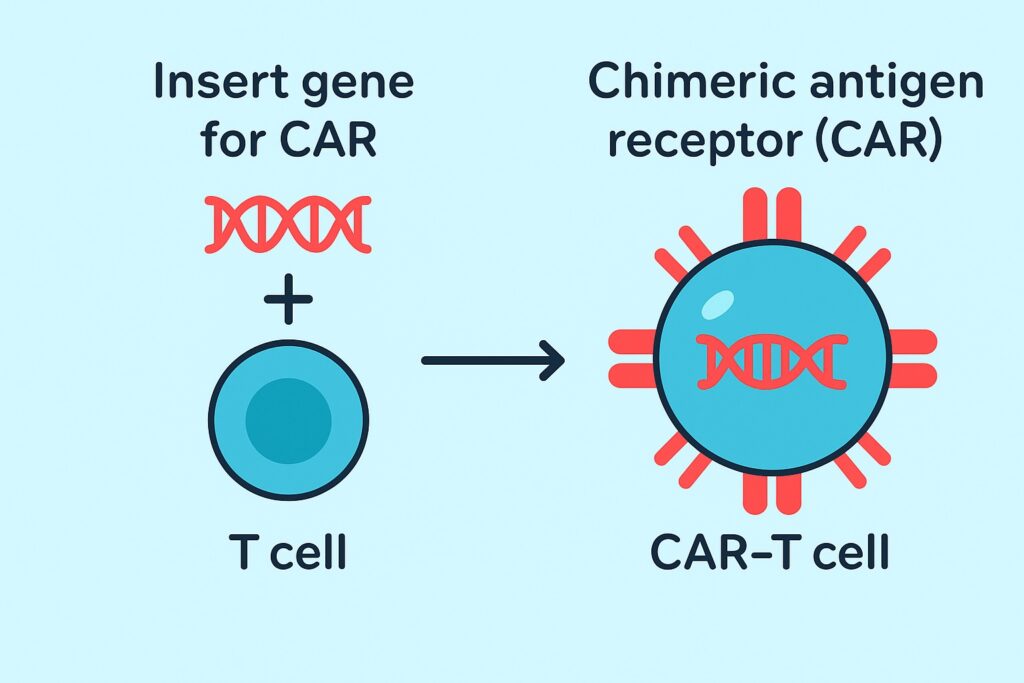
What Are CAR-T Cells?
CAR-T cells, short for Chimeric Antigen Receptor T cells, are immune cells engineered to recognize and attack cancer cells specifically. Unlike regular T cells, which have difficulty identifying cancer cells, CAR-T cells are equipped with artificial receptors (CARs) that allow them to target specific antigens (e.g., CD19).
The CAR structure typically consists of three key domains:
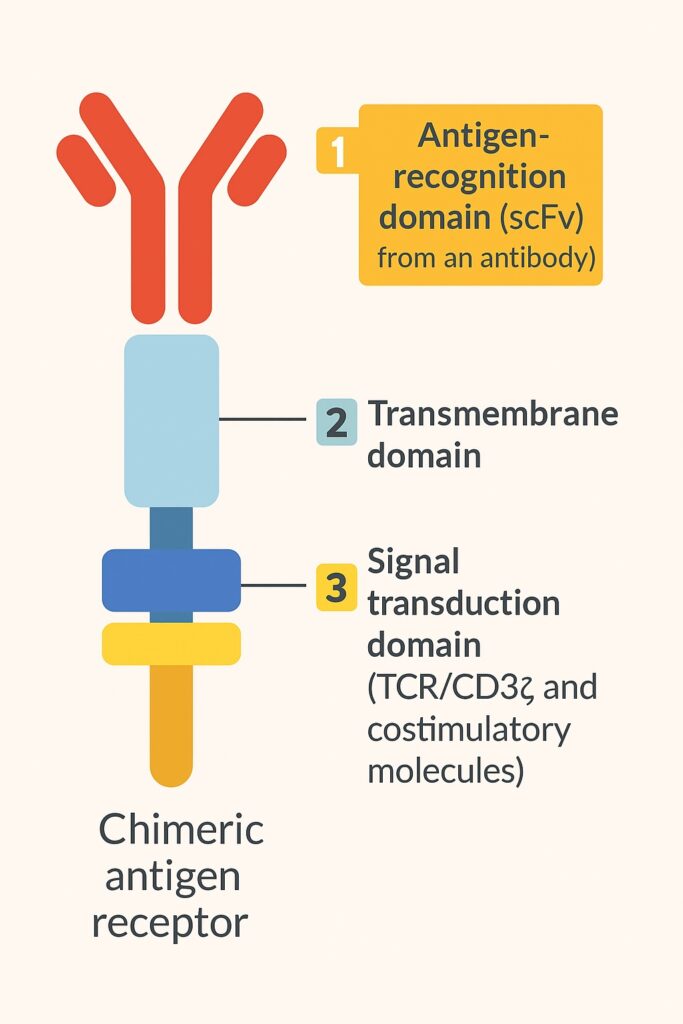
- Antigen-recognition domain derived from antibodies (scFv)
- Transmembrane domain
- Intracellular signaling domain (e.g., TCR/CD3ζ and co-stimulatory molecules)
Thanks to this structure, CAR-T cells can bind to target cancer cells and deliver strong cytotoxic signals. Currently approved CAR-T therapies mainly focus on blood cancers, but research is advancing for solid tumors as well.
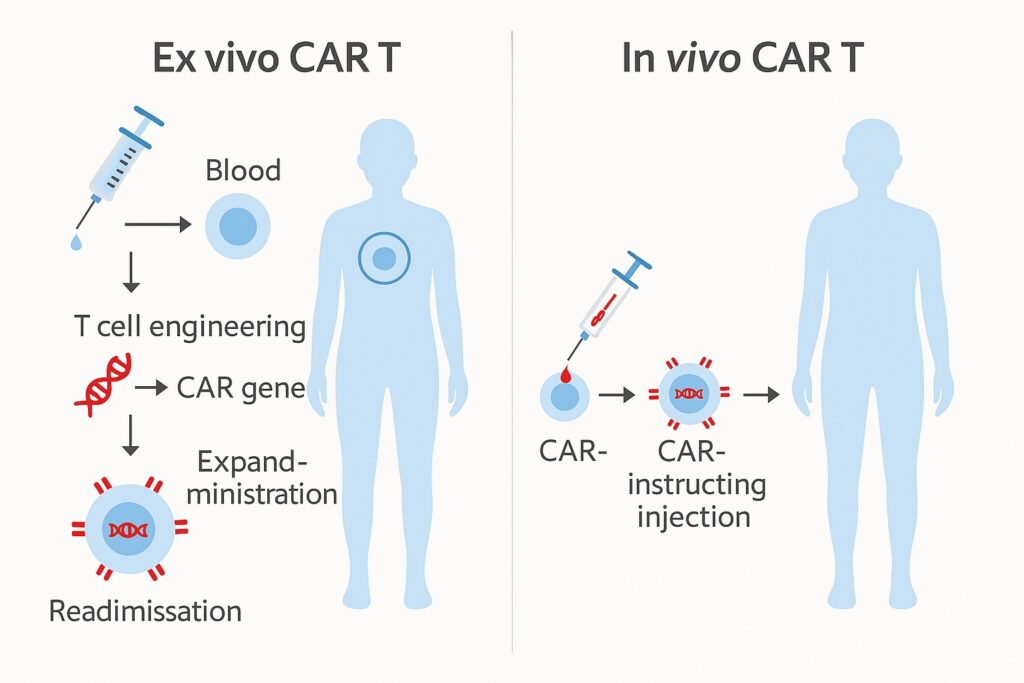
3. Ex Vivo vs. In Vivo CAR-T: What’s the Difference?
The dominant approach today is the ex vivo CAR-T therapy, in which T cells are extracted from the patient, genetically modified outside the body to express CARs, and then reinfused. In contrast, the emerging in vivo CAR-T method involves delivering CAR genes or reagents directly into the patient’s body, converting native T cells into CAR-T cells in situ—without the need for external cell processing.
In vivo CAR-T offers potential advantages such as reduced cost, simplified manufacturing, and broader patient accessibility. Although still largely experimental, it holds great promise for the future.
4. Summary and Coming Up Next
Emily’s miracle showed the world the power of CAR-T therapy. Now, multiple CAR-T products are approved, and the innovation continues. In our next article, we’ll dive into the technical strategies behind in vivo CAR-T and explore the latest efforts to bring it into clinical reality.
🔗 Related Articles & Series Links











Comments