What Breaks in CAC? — Human Evidence on Appetite, Metabolism, Fat & Muscle
In Part 4 we look behind the scenes using human data: central appetite circuits, systemic metabolism, adipose tissue, and skeletal muscle. We map these mechanisms back to the phenotypes from Part 3 (fat-first, muscle-first, mixed) to guide practice.
3 takeaways
- Central appetite brakes (e.g., GDF15–GFRAL) plus inflammation jointly suppress intake and rewire metabolism.
- Metabolic tilt—more gluconeogenesis, insulin resistance, and lower mitochondrial efficiency—yields a net breakdown > synthesis.
- Adipose lipolysis and muscle proteolysis reinforce each other, accelerating composition loss and functional decline.
1) Central appetite control: why eating feels hard
In patients, circulating GDF15 often rises and signals via GFRAL in the brainstem, driving anorexia and nausea. Hypothalamic melanocortin imbalance (POMC/AgRP), neuroinflammation from IL-6/TNF-α, leptin resistance, and blunted ghrelin responses also appear in human studies.
- Clinic link: Poor appetite/nausea scores tend to track early SAT/VAT loss on CT.
- Trialable idea: In GDF15-high, anorexia-dominant patients, anti-GDF15 strategies plus antiemetics and energy-dense diets may gain the most.
2) Systemic metabolism: fuel flows change
Human tracer/PET work shows hepatic gluconeogenesis up, peripheral glucose uptake down, and fatty-acid mobilization up. Resting expenditure may rise in subsets. Mitochondria become less efficient (ΔΨm↓, ROS↑), pushing synthesis down.
Clinic link: Rising fasting glucose/HOMA-IR often co-travel with muscle loss and fatigue—explaining why nutrition alone can be insufficient.
3) Adipose tissue: lipolysis leads
Subcutaneous and visceral fat exhibit heightened lipolysis via ATGL/HSL; circulating NEFAs climb. Evidence for browning is inconsistent in humans; loss of quantity dominates. Catecholamines and inflammatory tone sustain the draw-down.
- Clinic link: Patients with early SAT/VAT loss often report worse appetite and nausea.
- Action: Prioritize antiemetics/appetite support and energy replacement; close the burn > supply gap fast.
4) Skeletal muscle: breakdown wins, synthesis loses
Biopsies and circulating markers show higher ubiquitin–proteasome (MuRF1/Atrogin-1) and autophagy, while mTORC1 is suppressed by inflammation and energy stress. Mitochondrial inefficiency (lower complex activity/PGC-1α) and slower repair, plus fragile neuromuscular junctions, compound weakness.
Clinic link: CT SKM loss + low radiodensity tightly tracks chair-stand and stair decline.
Action: Resistance training + protein-forward nutrition (EAA/leucine) to lift synthesis; fix pain/sleep/inactivity in parallel.
5) Crosstalk with gut, liver, and tumor
- Gut: Barrier leak and microbiome shifts → LPS-driven inflammation (IL-6 up).
- Liver: Tumor/inflammation push gluconeogenesis and lipid rewiring → worsens peripheral fuel scarcity.
- Tumor factors: Cytokines, exosomes; PIF-like activities have been reported but remain inconsistent in humans.
6) Biomarkers: who fits which pattern?
Combining CRP/IL-6, GDF15, albumin, leptin/adiponectin ratio, and insulin-sensitivity indices with CT, function, and PROs helps cluster patients into anorexia-dominant, inflammation-dominant, or metabolic-resistant phenotypes—actionable categories for trials and care.
7) Mechanism–phenotype lookup (clinic quick map)
Fat-first
- High GDF15 / poor appetite
- Early SAT/VAT drop
- First move: Antiemetics + appetite/energy support; dietitian early.
Muscle-first
- Inflammation + insulin resistance
- SKM drop + low radiodensity
- First move: Resistance + protein; rehab; fix pain/sleep; review catabolic meds.
Mixed
- High CRP/IL-6; severe fatigue
- Fat and muscle fall together
- First move: Multi-modal early and stronger; escalate symptom care.
8) The causal loop
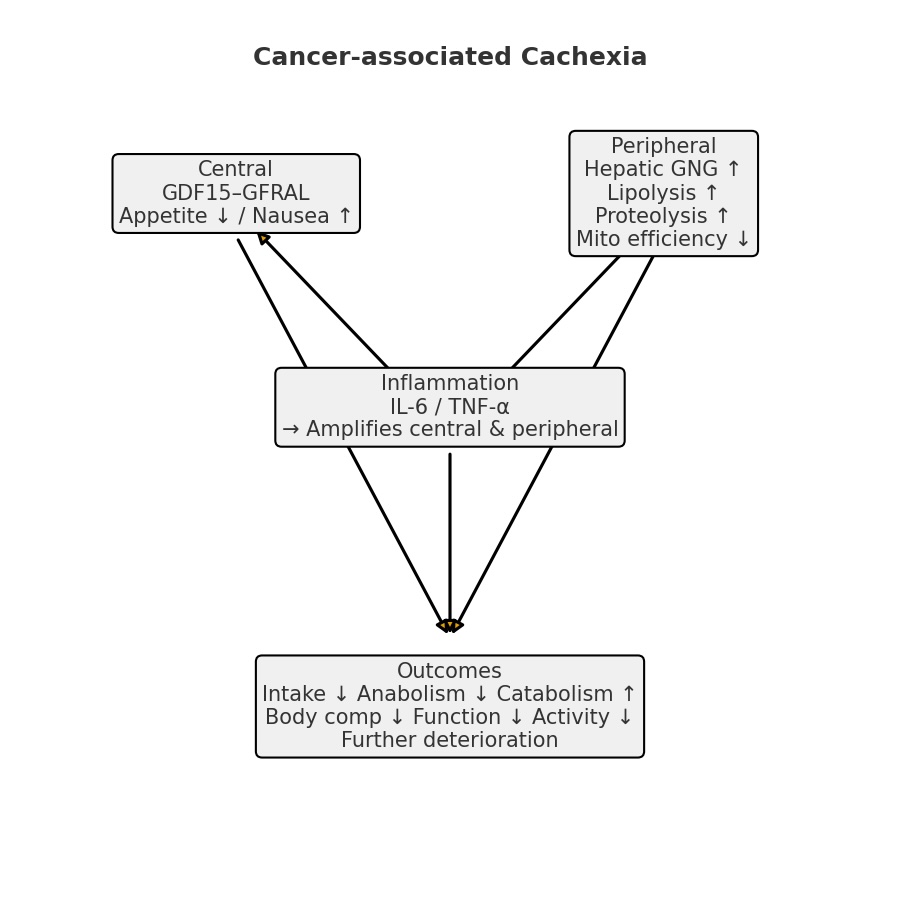
- Central: GDF15–GFRAL → appetite↓/nausea↑
- Peripheral: Gluconeogenesis↑, lipolysis↑, proteolysis↑, mitochondrial efficiency↓
- Inflammation: IL-6/TNF-α amplify both central and peripheral changes
- Outcome: Intake↓ + synthesis↓ + breakdown↑ → composition↓ → function↓ → activity↓ → further decline
The clinical question is which gear to stop first.
My Perspective
Decisions improve when we identify phenotype × mechanism early. An anorexia-dominant patient (high GDF15, early SAT/VAT loss) calls for antiemetics/appetite/energy measures first; a muscle-first patient (inflammation/IR, SKM loss) benefits most from resistance training, protein strategy, and symptom fixes that unlock activity. Human evidence points to a practical rule: decide fast which cog to block, then iterate.
Next in the Series
Part 5: “What Works Today — Nutrition, Exercise, Anti-inflammation, and Current Drugs.” We’ll translate mechanisms into a clinic workflow, including diet/exercise prescriptions and how to position agents like anamorelin and low-dose olanzapine.
Edited by Morningglorysciences
Related Article












Comments